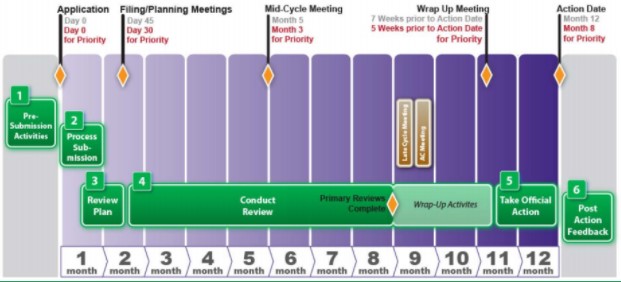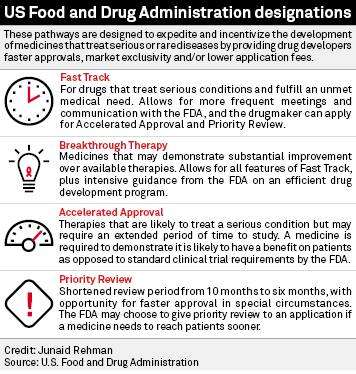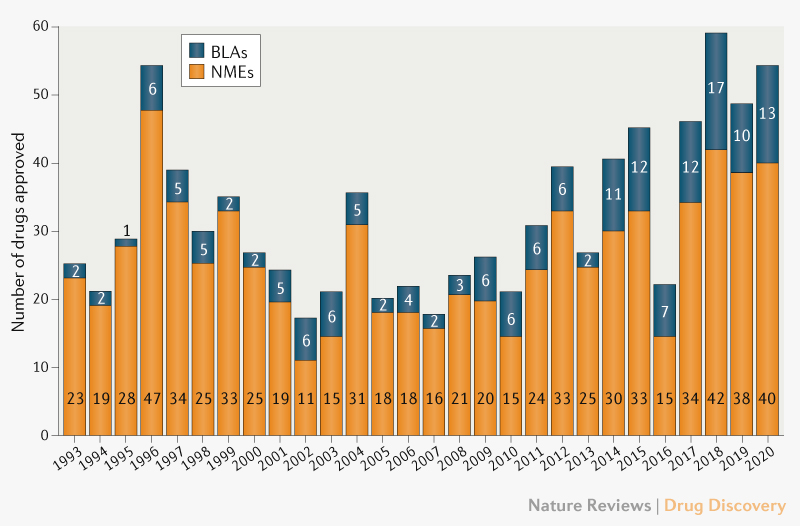
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha
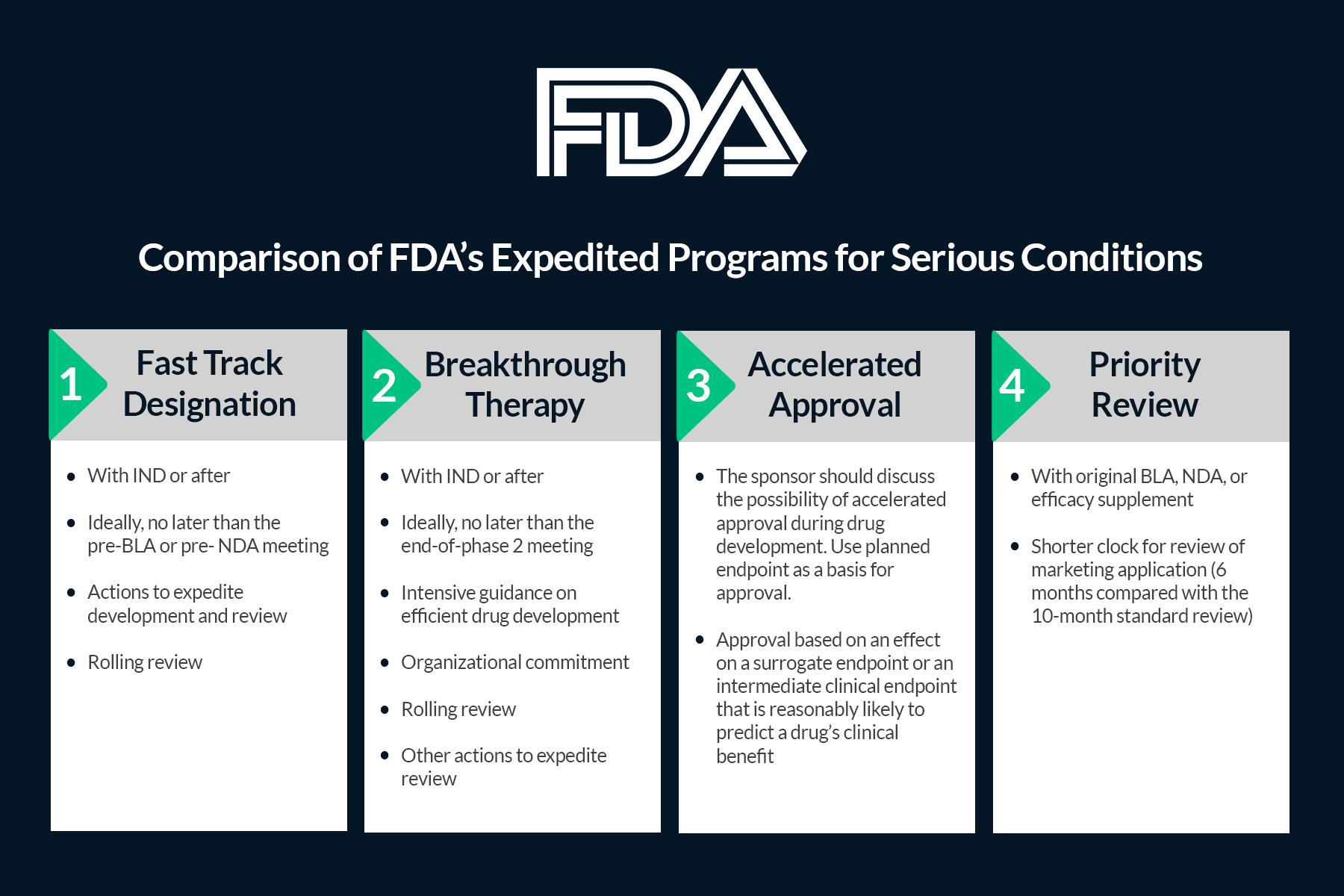
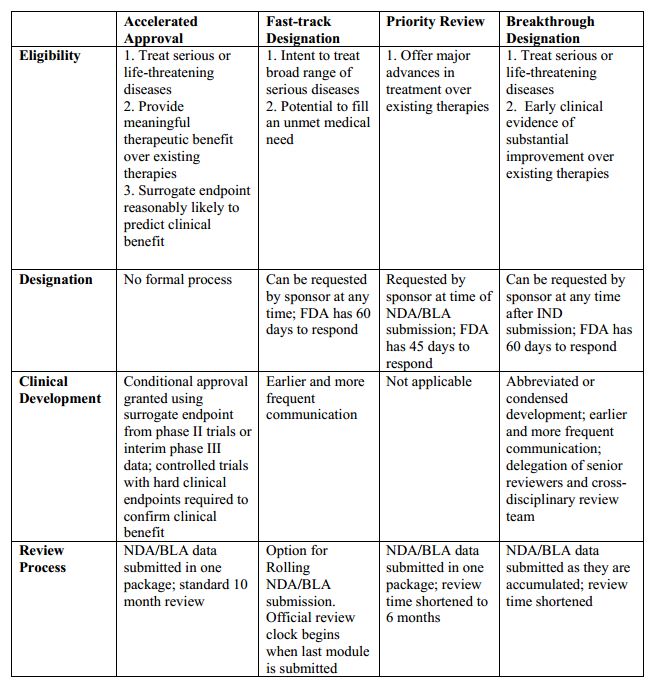
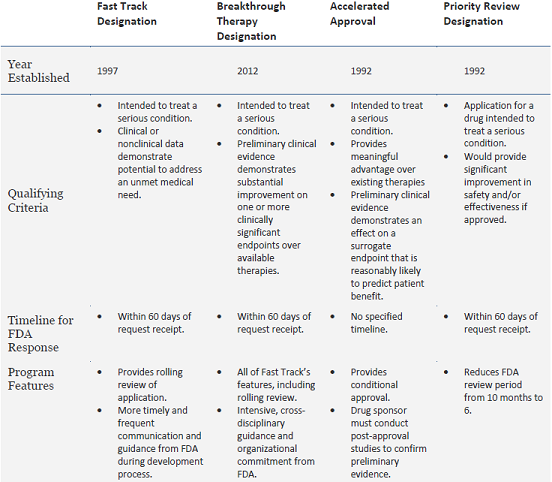
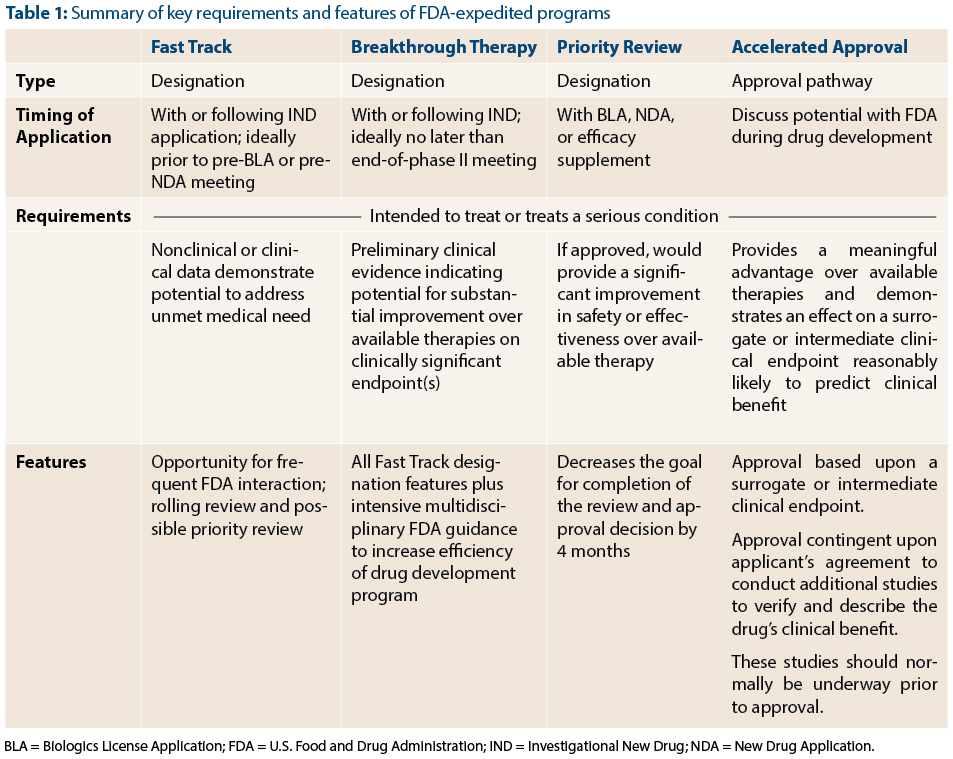
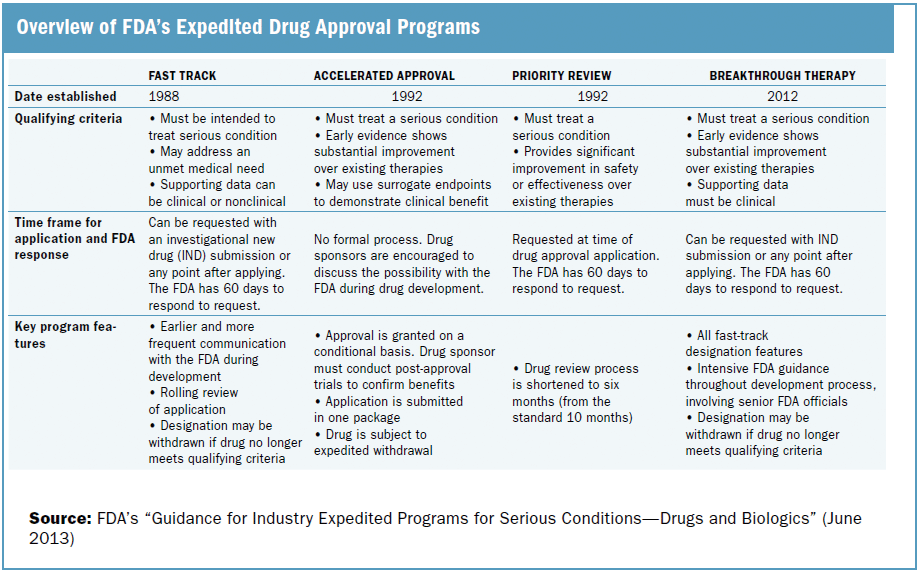
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group

Y-mAbs Reports Initiation of Rolling Review of BLA for Naxitamab to the US FDA to Treat Neuroblastoma

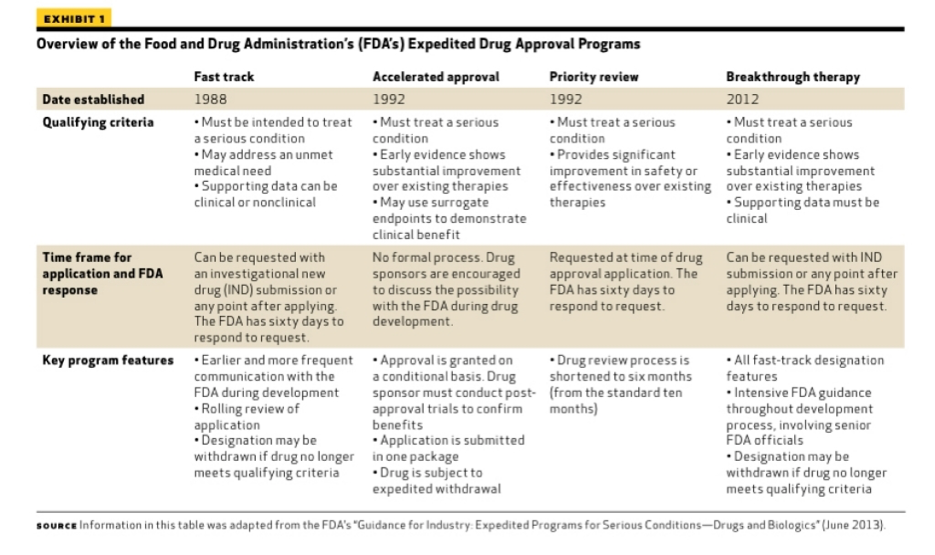
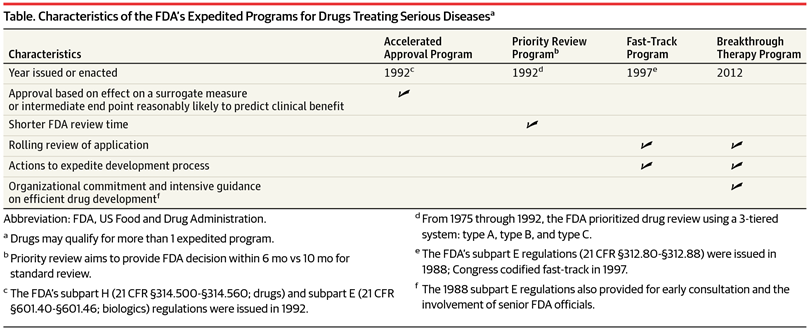
A detailed analysis of expedited regulatory review time of marketing authorization applications for new anticancer drugs in the US and EU - da Costa Gonçalves - 2022 - Clinical and Translational Science - Wiley Online Library
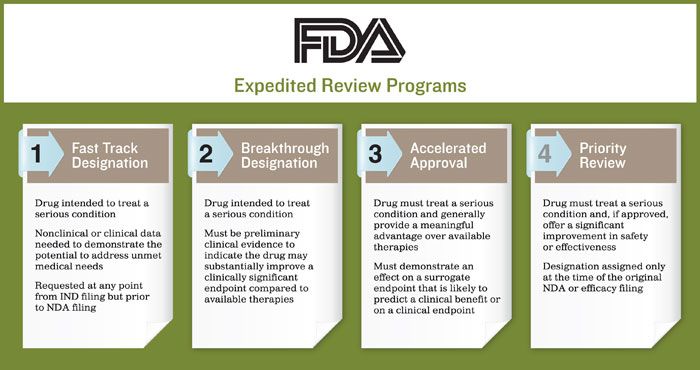
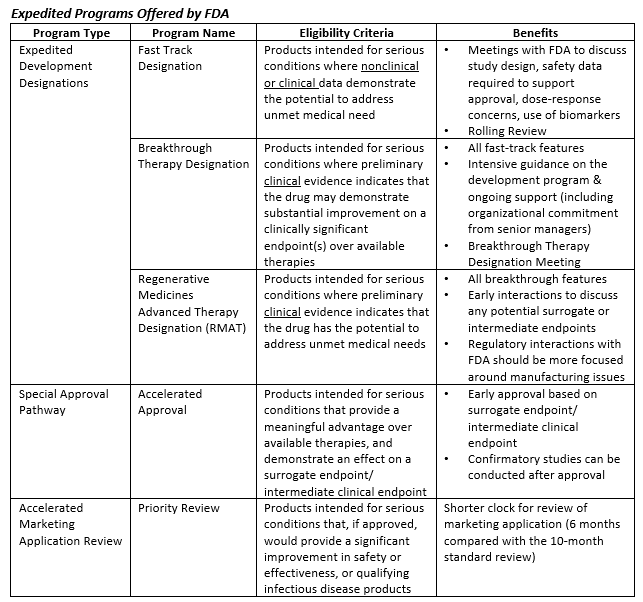
FDA Moves Against Fast Track Vaccines - News about Energy Storage, Batteries, Climate Change and the Environment